Cold tolerance
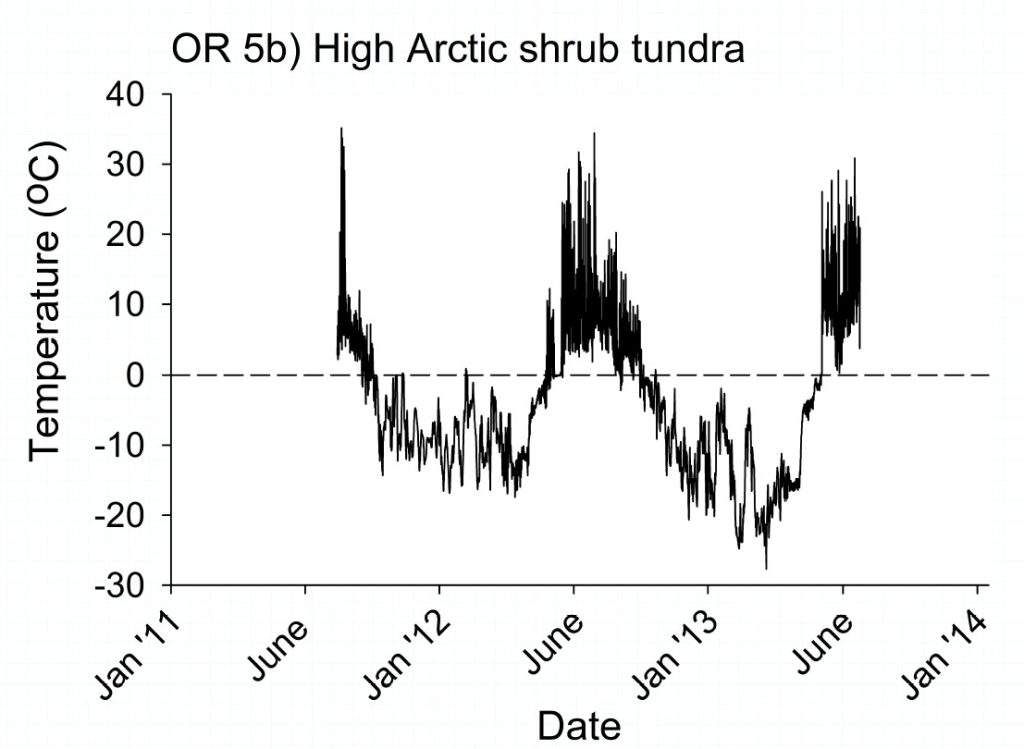
For the invertebrates on Svalbard winter lasts for almost 10 months of the year (Convey et al. 2018). In the majority of winters the ground begins to freeze in September and does not thaw until June the following year. During this time the invertebrates are at environmental temperatures either under rocks and stones, attached to vegetation or, more often, within the soil. Unlike mammals such as the Svalbard reindeer, which maintain an elevated core body temperature using a thick insulative layer of fat and fur to insulate against the loss of heat from metabolism, invertebrates by and large have a body temperature similar to the environment.
Prof Pete Convey, polar ecologist, BAS.
Click here for a BBC radio programme on life in polar regions and an interview with Prof. Pete Convey (British Antarctic Survey) on polar invertebrates.
Invertebrate cold tolerance strategies
This means that for invertebrates frozen in the soil they have to cope with body temperatures that can fall below -30ºC as well as extreme desiccation and anoxia. During the winter many polar invertebrates are extremely cold tolerant, many species surviving temperatures below -60ºC. And this is with a body temperature of -60ºC. Many polar explorers have learnt to their cost the problems humans have once their extremities have become frost bitten, for example Robert Peary who, during one of his attempts on the North Pole at the turn of the 19th century, removed his leather boots and found his toes stayed in the boot or Ranulph Twistleton-Wykeham Fiennes who after returning from crossing the Arctic on foot amputated the tips of his own frost bitten fingers in his garden shed with a saw. However, polar invertebrates do not have this problem and once cold hardened and frozen in the soil they are remarkably tolerant, many species able to survive over four years at below -20ºC. The question then is, how do they accomplish this feat? Basically these invertebrates can be said to use one of three strategies. However, as is often the case when trying to impose an artificial hierarchy on a natural system there are few clear cut boundaries and invertebrates may use a more than one strategy.
Unable to effectively migrate the invertebrates have to tolerate the thermal regime of Svalbard throughout the year. Once the autumn arrives the soil dwelling animals are frozen into the ground and exposed to environmental temperatures. This can result in extended exposure to sub-zero temperatures (see the figure taken from Convey et al. 2015).
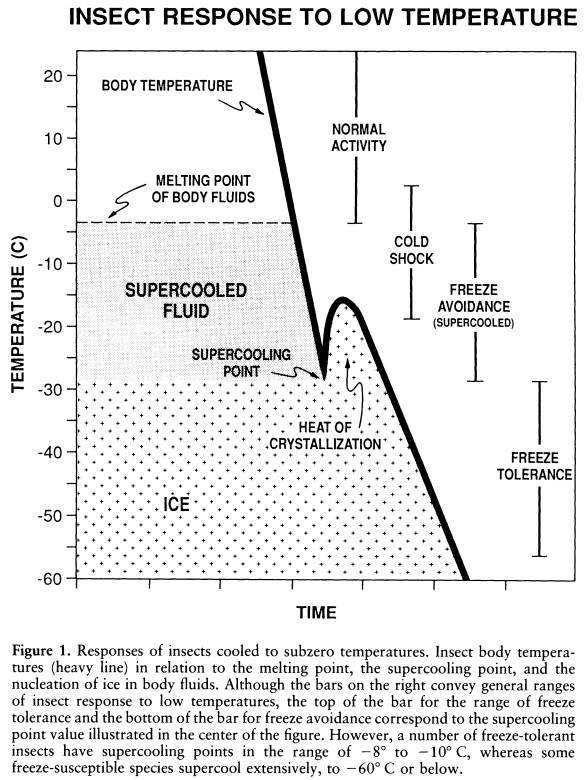
Damage due to low temperature is caused by many factors, one of which is the freezing of free water in the body. As temperatures decline below 0ºC the chance of water present freezing increases. Such ice is dangerous and may kill the animal. In such cases the invertebrate has three obvious choices: prevent the water in the body from freezing, survive the formation of this ice within the body or remove the water from the body. These physiological strategies are termed Freeze Avoiding, Freeze Tolerance and Dehydration (or desiccation) respectively.
Once fully cold hardened these animals are extremely cold tolerant, surviving four years at below -20ºC or below -60ºC. However, success in the Arctic is more than just overwintering survival and species have also adopted strategies that enable survival of short cool summers, for example the modified life cycle of the endemic aphid Acyrthosiphon svalbardicum (Heikinheimo, 1968) (Strathdee & Bale 1995) or free-running life cycles requiring several years to complete as for the oribatid mite Ameronothrus lineatus (Sövik & Leinaas 2002a,b)
1) Freeze avoidance (susceptibility)
Freeze avoiding species are defined as those which would die should they begin to freeze. These classes of invertebrates depress their freezing temperature, the supercooling point (SCP), and when fully winter cold hardened often possess SCPs below -20ºC. The majority produce cryoprotectants, often polyhydroxy alcohols such as glycerol or trehalose. Others also produce Antifreeze Proteins (Thermal Hysteresis Proteins) which stabilize the supercooled state by binding to incipient ice crystals and preventing further growth.
The endemic Svalbard aphid A. svalbardicum overwinters as an egg. The life cycle is finely tuned to the short Svalbard summer to ensure the presence of sexual morphs in late summer and the production of eggs before winter. These eggs are extremely cold tolerant with a mean SCP of -36.2 ºC and -38.2 ºC dependent on acclimation temperature. The need for such high levels of cold tolerance become apparent when the overwintering site is considered. The eggs are attached to the leaves of Dryas octapetala L. along ridgelines or at the top of slopes where there is only a thin insulating snow lie since the winds sweep the areas clear. This results in the eggs being exposed to the low air temperatures close to the ground during the polar winter, temperatures which can on occasion fall below -30°C. However, the advantage of inhabiting areas with limited snow accumulation is that they are also among the first areas to clear of snow the following spring and hence have the longest duration of summer.
1) Freeze tolerance
Freeze-tolerant species survive the freezing of extracellular body water and often synthesize specialist ice nucleating proteins in their haemolymph to ensure freezing is initiated in this tissue. For most freeze-tolerant species intracellular freezing is lethal and freezing is restricted to extracellular water. Freeze-tolerance has evolved several times in different insect orders, probably in response to different environmental challenges. In the northern hemisphere freeze-tolerance enables insects to survive extended exposure to low temperature while in the southern ocean the strategy enables activity during mild winter periods without the need for extensive cold hardening. Many freeze-tolerant species have a relatively high SCP around -7ºC. Once ice formation has been triggered in the haemolymph water is drawn out of the cells in response to the increasing osmolality of the haemolymph due to the growing ice crystals removing the liquid water. This desiccates the cells in turn increasing their solute concentrating and so depressing the freezing temperature of the intracellular matrix and inhibiting freezing. Overwintering in this semi-frozen state has several advantages, the risk of lethal intracellular freezing is reduced, metabolic rate decreased and body fluids are conserved (compare with desiccation strategy below).
The heleomyzid dipteran Heleomyza borealis (Boheman, 1865) overwinters as a final stage larva and is common in the ornigenic debris under bird colonies on Svalbard, especially black-legged kittiwake (Rissa tridactyla). Here winter temperatures are typically average -10oC but may decline significantly further on occasion since the overwintering habitat is under the wind scoop at the base of the cliffs and is not often covered by an insulating snow layer. When temperatures rise in the following spring the animal pupates with the adults emerging a few weeks later. The overwintering larvae is freeze-tolerant with a SCP of -7oC. Once in this frozen state the animal is extremely cold tolerant, 80% of the larvae survived exposure to -60oC (survival recorded 120 days after exposure) (Worland et al. 2000). At -60oC, 81% of the body water is frozen, the remaining being the osmotically inactive water.
The ability to withstand freezing is not limited to invertebrates; several species of vertebrate have also evolved freeze tolerance. Examples include painted turtle hatchlings and garter snakes but amongst the best researched is the wood frog. In this video (click here) Prof John Costanzo, one of the leading researchers in freeze tolerance demonstrates this ability. Video is now quite old and the quality is not the best but it is presented by Costanzo.
1) Freeze desiccation
The third strategy, desiccation, was first observed in overwintering earthworm cocoons and later demonstrated in Collembola. The strategy relies on the fact that Collembola have a permeable cuticle and overwinter in pores in the frozen soil (Worland et al. 1998, Holmstrup & Sömme 1998, Holmstrup et al. 2002). At high sub-zero temperatures the animal remains unfrozen by virtue of its solute concentration when the soil has frozen. Since the vapour pressure of liquid water is greater than ice there will be a net movement of water vapour from the animal to the surrounding ice and the animal will desiccate until the vapour pressure of the body fluids equals that of the atmosphere. The SCP of the desiccated tissues thus decreases and the animal will not freeze. In principle, this strategy is akin to freeze-tolerance except that with freeze-tolerance the frozen water remains in the animal whilst in desiccation the water freezes outside the body. As the soil warms up during spring the relative humidity of the air surrounding the collembolan increases and the animal rehydrates and eventually regains activity.
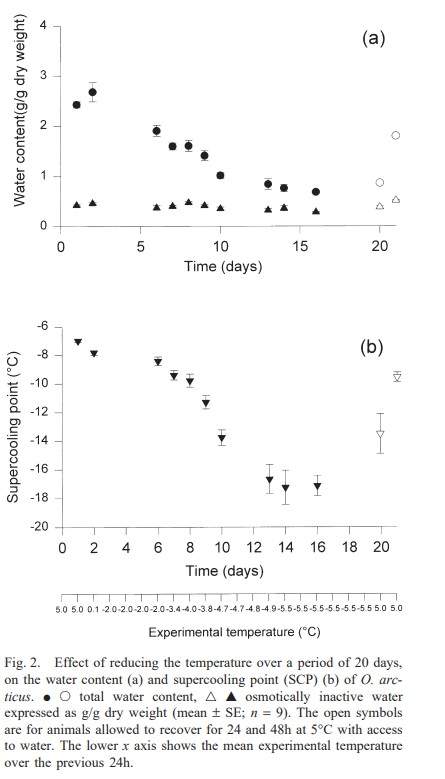
Megaphorura arctica (Onychiurus arcticus) (Tullberg, 1876) (Collembola, Onychiuridae) is a large collembolan that is common beneath birdcliffs where it can be collected in large numbers by pooter from under stones. It is a species almost exclusive from high Arctic areas, recorded also from northern Norway and Iceland, whose general distribution is northern Palaearctic although there are records from as far south as the UK. On Svalbard the ground is frozen between September and June and for much of the period is at -10oC. Initial studies determined a summer SCP of around -6.1oC and that the species is freeze-avoiding. Like all Collembola the cuticle is freely permeable to water vapour and desiccation in a dry atmosphere is rapid and lethal with the animal loosing >60% of their body water in less than one hour (Worland et al 1998). However the paradox of a freeze-avoiding with a high SCP and which dies if desiccated was resolved by studies on the manner of desiccation, that of slow desiccation in the presence of ice. In this desiccated state the animals can survive extended periods at sub-zero temperatures.
Adaptation to the Arctic: Life cycles
Arctic regions are characterized by long cold winters and short cool summers. In order to establish invertebrates require more than to be able to tolerate the cold winter, they must also be able to grow and reproduce during the cool summer.
Many species have extended life histories where they grow a little during each summer until finally adult. This strategy is common amongst soil invertebrates where animals such as the soil oribatid mites might take five years to become adult. A species of moth in Greenland, Gynaephora groenlandica, requires at least seven years as a larvae before it has grown sufficiently large to pupate and if the summers are poor it might require twice as long.
Other species have a short life cycle and can only overwinter as an egg for example the fairy shrimp, Lepidurus arcticus. These animals require cold, clean water that lacks fish predators. They overwinter as an egg and develop to adult during the short summer. An adult such as this can be up to three centimetres in length.
Another example with a delicately tailored life history to the Arctic environment are the two endemic aphids, Sitobion calvulus and Acyrthosiphon svalbardicum (Coulson et al 2014).
To understand how the Arctic aphids have adjusted their life cycles to suit Svalbard it is first necessary to understand the life cycle of the proginators of these aphids; the temperate aphids.
Aphids have two basic strategies; they are either holocyclic or anholocyclic. During the warm summer months the aphids are parthenogenetic viviparae, that is to say they are all females all giving birth to more females without the need to mate. Anholocyclic clones overwinter as these adult females but in northern European regions require a mild winter to survive. Adult aphids are generally not greatly cold tolerant. After mild winters these females begin to produce young as soon as temperatures rise the following spring and give rise to the aphid outbreaks known well to all gardeners. However, during colder winters it is only the egg that can survive. Clones overwintering via an egg are known as holocyclic. These clones produce eggs in the autumn and will hatch the following summer. Due to the time lag required for these eggs to hatch and for the young to mature, the build-up in the aphid populations is delayed in years following a cold winter. Production of these eggs in the autumn is dependent on environmental cues, often the change in day length. The aphid detects the shortening days of autumn and instead of producing more viviparae, the offspring are sexual males and females which mate and produce the overwintering eggs.
This requirement for a long summer means that the local distribution of the aphid is restricted by winter snow fall. Where snow accumulates the start of the following summer is delayed until the snow mass has melted. At a critical point the summer is too short for the aphids to complete their lifecycle before the onset of winter. Hence the aphid is restricted to areas with shallower winter snow and consequently colder winters since they lack the insulative blanket of snow.
In the Arctic the aphids have a problem. They cannot overwinter as adults. Nonetheless they cannot wait to produce sexual offspring until day length starts to change as by that time there is not enough summer remaining for this generation to mature and produce eggs before the winter arrives. The solution is to develop a genetically programmed lifecycle that is independent of the environment. The lifecycle of S. calvulus is as short as it can be. From the egg a morph known as a fundatrix hatches and this produces a mixture of males and sexual females from which the overwintering eggs are produced. Very few aphid species produce sexual morphs from the fundatrix and this is believed to be an adaptation to the short Arctic summer. Note that no environmental cues are involved but that the aphid has minimalistic life cycle guaranteed to produce eggs before the onset of winter. While this life cycle is well suited to Arctic regions, the ability to rapidly build up a population is limited. The number of eggs produced by the offspring from one egg is small and aphids on Svalbard have not only to survive the climate but also avoid a range of predators and parasitoids. The other endemic aphid, A. svalbardicum therefore has a strategy that ensures egg production every year but which also can boost the number of eggs produced. This aphid also has a life cycle similar to that of S. calvulus but has a second pathway. A few of the offspring of the fundatrix are not males or sexual females but are viviparous females as in temperate aphids. The offspring of these adults are always males and sexual females. This extra generation usually fails to produce eggs as the extra time required for the viviparae to mature before producing males and sexual females means that the winter arrives before the eggs are laid. Why then have this extra generation? The answer seems to be that by sacrificing a few adults in most years, in years where the viviparous generation succeeds to lay eggs the total egg production can be greatly enhanced. Making some basic assumptions and assuming no mortality it can be shown that in a year with a long summer the extra generation increases egg production several fold. Further, that this generation need only succeed once every 26 years for it to be evolutionarily advantageous to the aphid (Strathdee et al. 1993).
Despite this highly evolved Arctic lifecycle the aphid appears to locally limited in its distribution to locations where the summer is sufficiently long to complete the egg to egg lifecycle in the short summer; i.e., particularly warm locations or those where the snow clears early (Ávila-Jiménez and Coulson 2011).
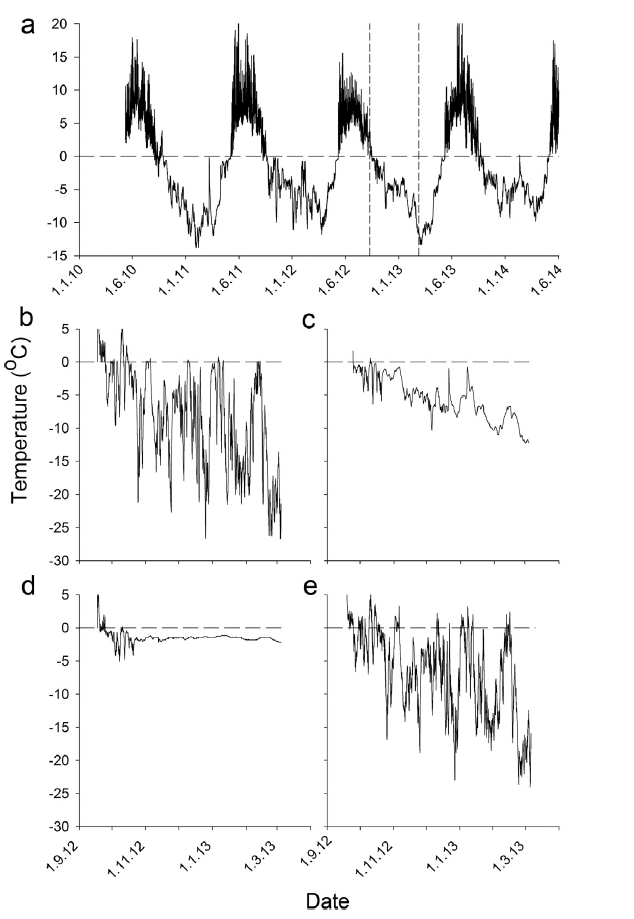
Example soil temperatures in a Dryas tundra (Endalen) (Convey et al. 2018 online electronic material). Note how warm the soil surface can reach far exceeding air temperatures and how winter soil temperatures vary depending on winter conditions and the interaction between air temperature and snow cover. Finally, that temperatures are constantly varying bringing into question the validity of using average (mean) temperatures to describe the conditions tye soil invertebrates experience both in summer and winter.
Convey et al. 2018
The deep windscoop at the kittiwake cliffs resulting in only a thin snow cover directly under the cliffs and hence no insulation from low air temperatures. Invertebrates overwintering here need to be able to tolerate temperatures approaching air temperature which can be well below -20°C.
Photo: Stephen James Coulson
Left: The habitat of M. arctica under the Stuphallet bird cliffs in Kongsfjord. Right: Removing a stone reveals large numbers of the collembolan.
The collembolan Megaphorura arctica (in older literature from Svalbard this species is referred to as Onychiurus arcticus) (Credit: Pål Hermansen)
Photos: Stephen James Coulson
- Ávila-Jiménez M.L & Coulson S.J. (2011) Can snow depth predict the distribution of the high Arctic aphid Acyrthosiphon svalbardicum (Hemiptera: Aphididae) on Spitsbergen? BMC Ecology 11:25
- Ávila-Jiménez, M.L., Solhøy, T., Gwiazdowicz, D.J., Fjellberg, A., Dózsa-Farkas, K., Monson, F., de Smet, W., Stur E., Ekrem T. and Coulson, S.J. (2019) The invertebrate fauna of Edgeøya, Svalbard: Arctic landscape community composition reflects pan-Arctic biogeography patterns Polar Biology, 42;837-850 https://doi.org/10.1007/s00300-019-02471-x
- Convey, P., Abbandonato, H.D.A., Bergan, F., Beumer, L.T., Biersma, E.M., Bråthen, V.S., D’Imperio, L., Jensen, C.K., Nilsen, S., Paquin, K., Stenkewitz, U., Svoen, M.E., Winkler, J., Müller, E. and Coulson, S.J. (2015) Survival of rapidly fluctuating natural low winter temperatures by Arctic soil invertebrates. Manuscript resulting from project on UNIS masters/Ph.D. Arctic Winter Ecology course (AB329/829) 2012-13 Journal of Thermal Biology 54, 111–117 https://www.doi.org/10.1016/j.jtherbio.2014.07.009
- Coulson, S.J., Convey, P., Aakra, K., Aarvik, L., Ávila-Jiménez, M.L., Babenko, A., Biersma, E., Boström, S., Brittain, J., Carlsson, A.M., Christoffersen, K.S., De Smet, W.H., Ekrem, T., Fjellberg, A., Füreder, L., Gustafsson, D., Gwiazdowicz, D.J., Hansen, L.O., Holmstrup M., Kaczmarek, L., Kolicka, M., Kuklin, V., Lakka, H-K., Lebedeva, N., Makarova, O., Maraldo, K., Melekhina, E., Ødegaard, F., Pilskog, H.E., Simon, J.C., Sohlenius, B., Solhøy, T., Søli, G., Stur, E., Tanaevitch, A., Taskaeva, A., Velle, G. Zawierucha, K. and Zmudczyńska-Skarbek, K. (2014b). The terrestrial and freshwater invertebrate biodiversity of the archipelagoes of the Barents Sea; Svalbard, Franz Josef Land and Novaya Zemlya. Soil Biology and Biochemistry 68; 440-470.
- Morewood, D. & Ring R.A. (1998) Revision of the life history of the High Arctic moth Gynaephora groenlandica (Wocke) (Lepidoptera: Lymantriidae). Can. J. Zool. 76;1371-1381
- Strathdee A.T., Bale J.S., Block W.C., Webb N.R., Hodkinson I.D. & Coulson S.J. (1993) Extreme adaptive life-cycle in a high arctic aphid, Acyrthosiphon svalbardicum. Ecological Entomology 18; 254-258.