When thinking of animals on Svalbard you first perhaps think of the big cuddly ones, the reindeer, fox or the marine animals including polar bears, walrus, seals or perhaps the highly visible (and often noisy) seabirds. But, whereas there are only three species of terrestrial mammal on Svalbard and only c. 30 species of birds that regularly breed in the archipelago, there are over 1,300 species of terrestrial or freshwater invertebrate known from Svalbard (Coulson et al 2014). And this is only a start. The true number is likely to be greater as only the fauna of the west coast, primarily from Isfjord and Kongsfjord, has been studied in any degree of detail. What’s more, they are here in Svalbard year-round, often able to tolerate body temperatures to below -30 °C (Coulson et al. 2023), and occur in great abundance; >300,000 per m² is not uncommon (Hodkinson 2013). However, since most invertebrates are small and often require specialised equipment to collect and observe it is easy to overlook this diversity.
Coulson et al. 2014 presents the most complete summary of the invertebrate fauna of Svalbard. Although it requires updating and information concerning the groups omitted from the survey, it remains largely an accurate description of the state of knowledge of the fauna of Svalbard. For an up-to-date review of the state of research and the terrestrial flagship of the Ny-Ålesund international research station see Pedersen et al. 2022.
For more recent revisions of specific groups see Stur & Ekrem 2020 (Chironomids), Seniczak & Seniczak 2020, Seniczak et al. 2020 (oribatid mites), Brittain et al. 2020 (freshwater invertebrates (in Norwegian)) and Dimante-Deimantovica et al. 2018 (freshwater Crustacea).
Invertebrate
Invertebrate is a general term that covers all animals that lack a backbone and hence includes everything from worms to insects, spiders, and, Crustacea.
The terrestrial invertebrate fauna of Svalbard may be amongst the most comprehensively catalogued for any region of the Arctic. There are over 700 articles in international journals considering this fauna. The current species inventory for Svalbard is under constant revision due to the large number of synonyms, taxonomic problems, and the inclusion of new species, either to Svalbard or new to science. But it is clear that, contrary to first appearances, Svalbard has a diverse invertebrate fauna which includes endemics, for example the aphid Acyrthosiphon svalbardicum (Heikinheimo 1968).
Abundance
Abundance is poorly known. Densities in one habitat type can vary greatly over very small local distances and hence accurately determing densities requires considerable sampling effort. Nonetheless, some data do exist and densities can be extremely great where Collembola can attain local abundances of almost 500,000 individuals per m².
Lack of geographic knowledge
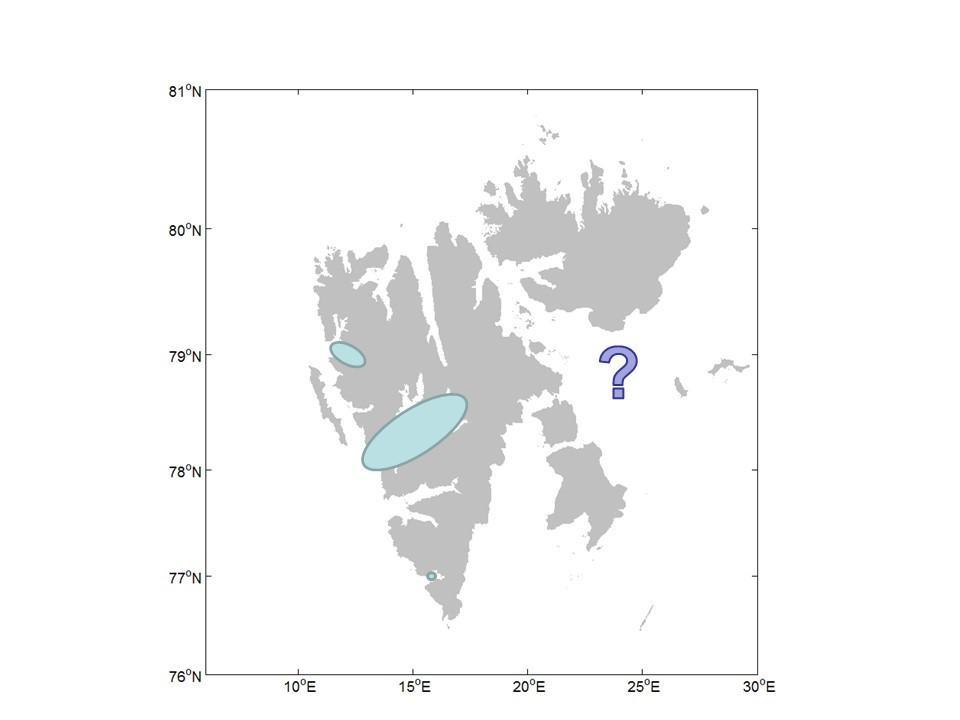
While a general understanding of the terrestrial and freshwater fauna is increasing fast, the great majority of the studies have been undertaken on the west coast, primarily in the Isfjorden region close to Longyearbyen and from Ny-Ålesund (map). Less than ten manuscripts consider the invertebrate fauna of the eastern regions. But, despite the lack of knowledge of the invertebrate fauna of the eastern regions, there are indications of different communities here than those found on the west coast. A small study in 2019 (Avila-Jimenez et al. 2019) of invertebrates from the island of Edgeøya in the eastern region of Svalbard lists 16 of the 140 species identified as not being recorded previously from Svalbard. Of these 16 species, six are undescribed new species. The reasons for this difference are as yet unclear but may be related to different immigration histories. The east coast being influenced by the East Svalbard Current bringing driftwood from the great Russian rivers. On the west coast it is the West Spitsbergen Current that dominates, bringing warm water northwards from the Atlantic and hence potentially baring a dissimilar fauna from the East Spitsbergen Current. The expectation is that further studies on this coast of the archipelago will reveal yet more species new to Svalbard as well as new to science.
Invertebrates in Svalbard
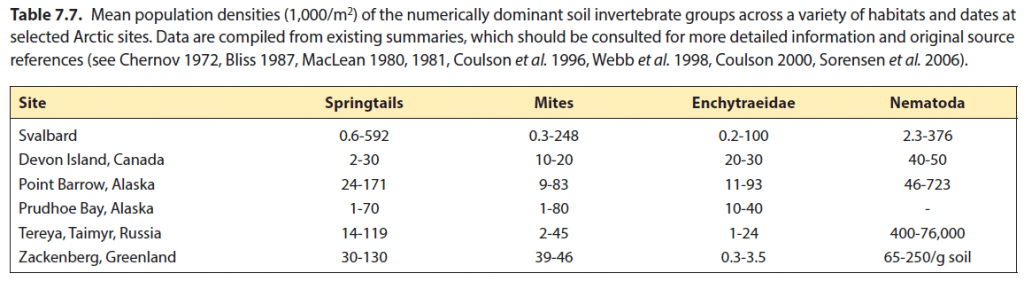
The invertebrates of Svalbard are a diverse group but information is really only available for a few groups including the Insecta (insects), Araneae (spiders), Acari (mites), Collembola (springtails), and Enchytraeidae (segmented worms). Even for these groups it is often species presence or absence that is recorded while knowledge of abundances is extremely limited and little or nothing known about population dynamics or phenologies. Indeed, the CAFF Status and Trends of Arctic Terrestrial Biodiversity Report 2021 (Aronsson et al. 2021) highlights just these problems and the lack of monitoring generally in the Arctic with the notable exceptions of Zackenberg and Nuuk in Greenland for which some population trends exist.
Class Arachnida
Sub-class Araneae
There are 15 recorded species of spider in Svalbard, 14 from the family Linyphiidae and one from the Gnaphodsidae. The spiders are all small black or dark brown animals with a maximum body length of a few millimetres. They are extremely common and can be seen under stones and logs, often remaining stationary for 4 or 5 seconds after the stone has been overturned before suddenly spring into life and escaping. It is also common to find the remains of egg cocoons or sometimes cocoons with unhatched eggs and the parent still guarding.
There are only a few records of spiders in houses. The species from Svalbard are adapted to the Svalbard environment and rarely enter houses.
A recent study showed that despite the liniphiids being considered ubiquitous, there are distinct communities depending on terrain aspect (Dahl et al. 2018).
Sub-class Acari
Some 140 species of mite are known from Svalbard. The majority are small innocuous animals living in the soil (picture) , however, some, such as the red species Neomolgus littoralis can be often seen running about over rocks on warm days, especially on the upper shoreline.
The mites can be divided into three groups, the hard bodied (oribatids) and the soft bodied (mesostigmatids and prostigmatids) mites. The hard-bodied mites have the greatest diversity on Svalbard with over 90 species recorded. However, and as with many other groups, many of these species have been only recorded once or have unclear identifications. These animals live in the soil and feed mostly on dead plant material or fungae. Although they are very small, often less than one millimetre long, they often live many years. One species had its life cycle described recently and was found to take five years to become adult (Søvik et al. 2023, Søvik & Leinaas 2003).
Less is known about the soft bodied mites. They are often predatory feeding on other mites (pictures) as well as other invertebrates. They also have a shorter life cycle although the life cycle on Svalbard is unknown.
The greatest diversity are soil dwelling. But others have specific environments, for example the parasite Dermanyssus hirundinis inhabiting the nests of snow buntings (where densities can exceed 26,000 individuals per nest, Gwiazdowicz et al. 2012) or Halolaelaps coulsoni in kittiwake guano (Gwiazdowicz & Teodorowicz 2017).
Other mites may exploit other animals in other means. For example, the gamasid mite Thinoseius spinosus uses Diptera to aid dispersal.
In the figure the mites can be seen intercalated under the abdominal segments where they are hard to remove.
In addition to the soil mites, the seabird tick Ixodes uriae present in Svalbard.
This species was relatively unknown in Svalbard (except for Bear Island) until recent but has become common on sea birds in Kongsfjord. The reasons for the arrival and rapid population increase are unclear but it has been linked to warmer winters and more ticks surviving in the cliffs where they overwinter and await the return of the breeding birds (Descamps 2013).
Class Collembola
The sprintails are a comparatively species rich group in Svalbard comprising of 79 species. Collembola are relatives of the insects, and may sometimes be seen in older taxonomies as an insect order but they are now recognised as being sufficiently distant as to be their own class.
Collembola are widespread and often highly abundant locally with densities in Dryas tunda approaching 500,000 per square metre. Nonethless, they show microhabitat preferences and communities can have distint compositions even at a local scale. On a regional scale several species have been collected from Edgeöya in the east that have not been seen despite extensive sampling on the west coast (Ávila‑Jiménez et al. 2019) indicating potential differences in immigration paths. Following on from this, five species of Collembola not seen elsewhere in Svalbard have been observed in the anthropogenic soils (often imported soil from Ukraine) in Barentsburg and a further two in Pyramiden indicating importation via human activities. One species, Deuteraphorura variabilis, may be invasive in the rich and characteristic nutrient-enriched communities beneath birdcliffs (Coulson et al. 2013).
The two most diverse orders of Collembola in Svalbard are the Poduromorpha and Entomobryomorpha (see images) while the globular-shaped Symphypleona, while locally abundant, includes fewer species.
Class Insecta
Order Coleoptera
A few beetles species occur in Svalbard. However, while as a global average the beetles comprise 38% of all insect species, in the Arctic beetles are far less diverse. Only 20 species are recorded from Svalbard forming 8.8% of the insect species in the archipelago and, in general, they are rare and difficult to find. However, common under stones beneath bird cliffs are two species of predatory rove beetles (Staphylinidae) which and prey on springtails (Collembola), particularly the large yellow springtail, Megaphorura arcticus (no common name) which is often the same size as the beetle. A third relatively common species is the weevil Isochnus flagellum occuring on the polar willow (Salix polaris) in, amongst other places, Adventfjord.
Order Diptera
As is common in the Arctic, the flies (Diptera), form the most diverse insect order. Perhaps the most noticeable being the non-biting chironomids numerous in the summer. These spend most of their lives as larvae before pupating and emerging as adults. Since the adults do not feed they have a short period as an adult. To conserve energy the males often wait on the ground until they hear a female fly past, responding to the sound of her wings. This behaviour was deduced by a Finnish biologist who observed that he could induce the males to take to the air by humming a Finnish folk tune (Syrjämäki 1968).
Mosquitoes (Aedes nigripes) occur in very high abundance in certain regions during the late spring/early summer, for example, Endalen, Adventdalen under Gruvefjellet, Kapp Thordsen and Brucebyen.
A commonly repeated story is that the mosquito arrived on Svalbard in 1918 with water barrels with phosphate miners from Tromsø. While this mosquito is indeed found on mainland Norway this story is unlikely to be true. This species was recorded by Holmgren during a scientific expedition to Spitsbergen in 1868 (Holmgren 1869) and was experienced in large numbers at Brucebyen by Victor Summerhayes and Charles Elton (Pond 2015) during their expedition in 1921 (Summerhayes & Spitsbergen 1923). As a brief aside, Elton was a professor at the University of Oxford and important in the development of ecology as a science (Pond 2015; wikipedia page). Indeed, it would be more surprising if the mosquito was not here prior to 1918 since it is a large flying insect already present on Greenland and mainland Norway, the distances involved are not great for such an insect and given correct wind directions could easily be blown here. See the observations of vagrant Lepidoptera in Svalbard such as the diamondback moth described elsewhere in Learningarcticbiology.info (and Coulson et al. 2002) later. As is often the case with Arctic mosquitos, this species doe not require a blood meal in order to develop and lay eggs. However, should a blood meal be obtained the egg production increases (Corbet and Danks 1975).
Order Lepidoptera
Usually one of the most obvious orders of insects are the butterflies and moths but on Svalbard there are only three species of resident moth and no butterflies. The Exile (Apamea maillardi) is observed in many locations, including Adventdalen (see image) but in extremely low numbers (see image). The other two are rarities; Pyla fusca (no common name) is only recorded from one location in Kongsfjord (Coulson et al. 2003) while second, Plutella polaris was first observed in Wijdefjord in 1873 but went missing until it was re-discovered by Geir Søli from the zoology museum in Oslo in 2015 (Søli et al. 2018).
Although only three are resident in Svalbard, that is they are able to complete their life cycle here, many species are occasionally blown here with the wind; vagrant species. Seven other species, including the green veined white (Pieris napi), Camberwell beauty (Nymphalis antiopa) and the painted lady (Vanessa cardui) have been observed in Svalbard (Kaisila 1973; Coulson et al. 2014). However, it is the diamondback moth, (Plutella xylostella) that is the best known vagrant. This species arrives regularly every few years, often in great numbers, after a period of southerly winds transporting it from the Norwegian mainland or Finland (Coulson et al. 2002). It cannot survive in Svalbard and soon dies or is predated.
Order Trichoptera
One species of caddisfly is present and can be seen in many ponds and lakes, for example Linnévatn or ponds in front of glaciers. This insect is best known for the case composed of small stones that the nymph constructs and lives in.
Order Hemiptera
Perhaps surprisingly there are three known species of aphid in Svalbard, two of which, and probably the third which remains unidentified to species as well, are endemic. The two named species have evolved unusual lifeccyles to cope with the Svalbard environment. The best known of these aphids is Acyrthosiphon svalbardicum (no common name) which feeds on Dryas octapetula, often found at the base of the leaves or on the flower shoots under the petals. This species is known from, for example, Endalen and the southern coast of Kongsfjord (Strathdee & Bale 1995). The lifecycle is complex requiring several generations each summer. Since this species can only overwinter as an egg and requires a specific duration of summer to complete this lifecycle and produce the eggs it is theorised that winter snow may limit its distribution and explain absence from otherwise apparantly suitable patches of the host plant (Strathdee & Bale 1995, Avila-Jimenez & Coulson 2011). The start of summer is delayed at locations where there is a deep snow accumulation the summer. At these locations the summer is of insufficient length for the aphid to complete its lifecycle before the onset of winter and hence the aphid cannot colonise these Dryas patches.
The second species, Sitobion calvulus, feeds on polar willow (polarvier) but occasionally also on Pedicularis sp. (hairy horsewort; lodnemyrklegg) (Gillespie et al 2007, discussed as Acyrthosiphon calvulus before the revision of the genus). Sitobion calvulus is only known from a location in Adventdalen and one more in Sassendalen.
The third species is a species of Pemphigus and is related to Pemphigus groenlandicus known from Greenland. As with P. groenlandicus, the species in Svalbard feeds on the roots of Poa sp.
As such, it is hard to find but is known from 14julibuka in Krossfjord and in Longyearbyen. Its distribution is probably much wider than this in Svalbard and likely occurs in many southerly facing slopes where Poa is present. This species appears to have a distinct genetic sequence compared to the Greenland species (Prof. Karina Wieczorek pers comm.) and further work is being performed to determine if the Svalbard individuals are P. groenlandicus or an undescribed new species.
A poster describing the three aphid species known from Svalbard can be found here: Wieczorek & Coulson poster.
Phylum Platyhelminthes
One of the few species of invertebrate introduced to Svalbard by man came with the accidental introduction of the Sibling vole (Microtus rossiameridinalis) (Henttonen et al. 2001; Fuglei et al 2008). The invertebrate goes by the name of Echinococcus multilocularis Leuckart, 1863 and has a complicated life cycle involving both the vole and the arctic fox (Alopex lagopus).
The parasite is an adult tapeworm in the gut of the fox. These adults produce eggs which pass out of the fox with the faeces and become attached the vegetation. The eggs can now be eaten by the vole while it is grazing on the grasses. Once in the gut of the vole the eggs hatch and the larvae migrate through the gut lining into the blood stream and to the liver. On arrival here they develop into a hydatid cyst, a large mass of tissue that swells and eventually kills the vole. The vole is then scavenged by the fox, eaten and the cysts develops into the adult tapeworm in the gut of the fox and is ready to produce more eggs. The parasite is potentially dangerous to humans since it is possible to become infected with the eggs. The subsequent illness is termed alveolar hydatid disease and is often fatal unless treated early (World Health Organisation information page). It is extremely difficult to surgically remove the hydatid cysts and main treatments rely on drugs that prevent development of the parasite. However, infection is rare. First one must ingest the eggs so normal hygiene procedures in known risk areas will reduce the likelihood of infection significantly. Also, in most cases the immune response of the body detects and removes the parasite. Should you become infected, given early detection, survival is now much improved with the 10 year survival rate being over 90%.
The vole is thought to have been brought to Svalbard with feed for the horses in the now derelict Russian mine at Grumont (Henttonen et al. 2001). Eggs of the parasite may be found where voles and foxes live in closed contact. Voles have established themselves along the coastline from Colesdalen to Adventfjord. It seems unlikely that the vole will be able to spread much further since the vole requires access to vegetation throughout the winter on which it feeds and a deep insulating snow layer to protect against extremes of low temperature. These conditions are found under the bird cliffs but are rare elsewhere. Since the foxes roam over large areas infected foxes may in principle be found almost anywhere but it seems the chances of being infected are low.
Phylum Crustacea
There are some 51 species of freshwater crustacean known from freshwater lakes and ponds on Svalbard (Brittain et al 2020). Perhaps the most obvious are Daphnia, water fleas, common in many ponds. This species is clonal, that is the population only consists of egg laying females. Individuals in Svalbard often have a high melanin content and so appear darker than individuals from more southerly locations such as the Norwegian mainland. This is considered an adaptation to help protect against high ultraviolet light levels (Hessen 1996). In some of the freshwaters you can find the tadpole shrimp, Lepidurus arcticus (Lakka 2013, Pasquali 2015, Pasquali et al 2019). This animal may be up to long as an adult and can be seen scuttling around on the mud at the bottom of the pond. In fact, only a small proportion of the population are visible since most will be hidden within the mud. This animal is considered a living fossil being unchanged for around 200 million years. They feed on dead organic matter but may predate the Daphnia and are cannibalistic, eating each other. Tadpole shrimps overwinter as an egg and develops rapidly into adults each year. They are typical of clean and cold freshwaters, especially those without fish. If fish are present then few tadpole shrimps can avoid being eaten unless there are shallow margins to the pond, areas to shallow for the fish to enter. In these shallows the tadpole shrimp may survive. Otherwise, the presence of tadpole shrimps is a good indicator that fish, i.e. arctic charr in Svalbard, are absent. They are also prey items for arctic terns.